The first batch of anti-COVID oral drug 2-DG, developed by the DRDO, was released on Monday by Defence Minister Rajnath Singh and Health Minister Harsh Vardhan.
The Drugs Controller General of India (DGCI) approved the 2-deoxy-D-glucose (2-DG) drug for emergency use as an adjunct therapy in moderate to severe coronavirus patients earlier this month.
In his brief remarks, Singh said the drug has brought a new ray of hope for the treatment of COVID-19 patients.
The Defence Ministry on May 8 said that the clinical trials of the drug 2-DG showed that it helps in faster recovery of hospitalised patients and reduces supplemental oxygen dependence.
The approval of the drug has come at a time India has been grappling with a record-breaking wave of coronavirus pandemic that has stretched the country’s healthcare infrastructure to its limit.
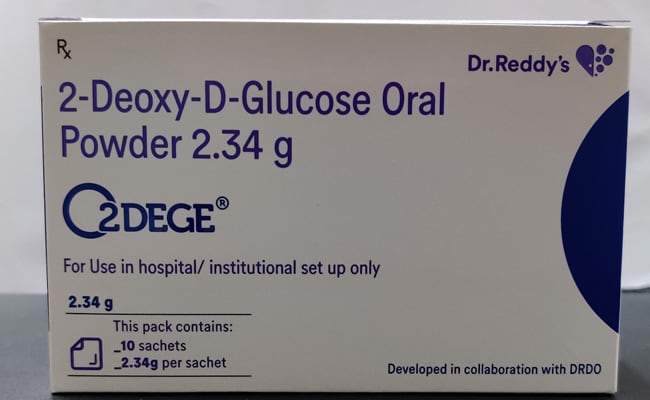
The anti-COVID-19 therapeutic application of 2-DG drug has been developed by the Institute of Nuclear Medicine and Allied Sciences (INMAS), a leading laboratory of DRDO, in collaboration with Dr Reddy’s Laboratories (DRL) in Hyderabad.
The drug comes in powder form in sachet and is taken orally by dissolving it in water, the ministry said.
In efficacy trends, the ministry said, patients treated with 2-DG showed faster symptomatic cure than the standard of care (SoC) on various endpoints.