The Serum Institute of India (SII), the world’s biggest vaccine maker, and Sputnik’s Indian distributor, Dr. Reddy’s Laboratories (REDY.NS), have both said they have approached health authorities about boosters.
Indian COVID-19 vaccine makers are lobbying the government to authorise boosters as supplies have so outstripped demand that one drugmaker told Reuters it had suspended a plan to produce more than 100 million doses of Russia’s Sputnik shot.
Follwoing WHO offical statement regarding Spread of Omicron variant in 57 Countries.,
India Health Minsitry sources has said its priority is to fully vaccinate all 944 million adults, though its immunisation experts are studying the need for boosters.
Indian government Portal on vaccine disclose it has given two doses to more than half of its adults and at least one dose to 86% of them.
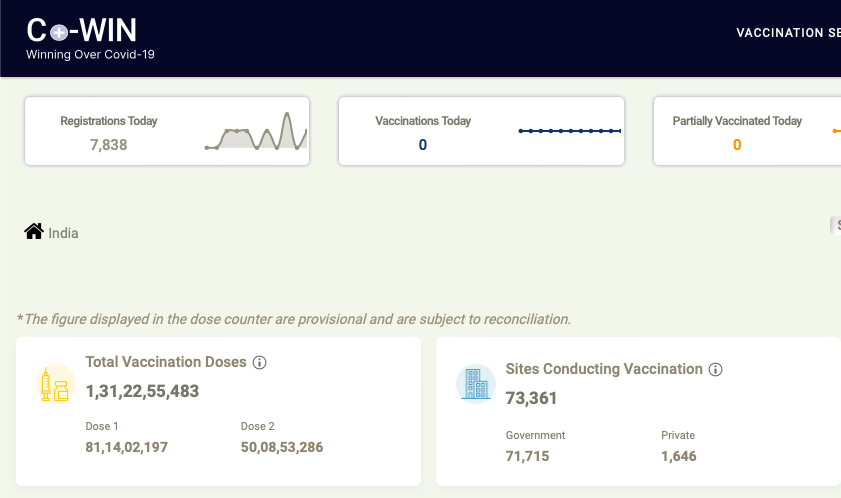
The government wants a total of 1.7 billion vaccine doses to fully immunise most of its adults, and the SII plans to make the last of its pending orders to meet its share of that demand by next week.
The SII has said it plans to halve its monthly output of Covishield, which has been 250 million doses, due to a lack of demand.
It is one of seven Indian companies that Russian sovereign wealth fund RDIF has struck deals with to make a total of nearly 1 billion Sputnik doses, both for export and for use in India.
But only 1.2 million doses of Sputnik V have been administered in India, government data shows.
Some 4 million doses, bottled in India using imported material from Russia, were exported, said two sources.
“We are talking to the regulator to allow it as a booster dose,” G.V. Prasad, managing director of Dr Reddy’s, told Reuters in a recent interview about the single-dose Sputnik Light.
“Right now, there’s no demand, the market is fully supplied by the Serum Institute. Internationally also, supply is not a constraint anymore.”
An Indian pharmaceutical company that was supposed to produce more than 100 million Sputnik doses has put the plan on hold without making a single commercial dose, said a source with direct knowledge of the decision.
The source, who declined to be identified in the interests of maintaining business relations, said the company had moved on to non-COVID products but would switch back to Sputnik if demand returned.
An RDIF spokesperson said its India partners would maintain their manufacturing plans.
“RDIF production partners in India will keep on manufacturing the same volumes of the vaccine as had been planned initially because we have always been primarily focused on export markets,” the spokesperson said in an email.
“At the same time, we expect Sputnik Light authorisation in India shortly, which would allow to use Sputnik Light as a booster in the country as well.”
The other vaccines either in trial or waiting approval in India are: Bio E’s protein sub-unit vaccine, Bharat Biotech’s nasal vaccine and Gennova’s mRNA vaccine.
The Indian government said in May it expected 460 million doses of the three shots between August and December, but no commercial production has begun.
Gennova says it expects demand to come from boosters, overseas markets and children when its shot hits the market after trials.
Bio E, Bharat Biotech, Cadila and India’s health ministry did not respond to requests for comment.
Vaccination for those under the age of 18 in India has not started yet, a market most of the companies are targeting next.









